If someone is in one of the ‘at risk’ groups or has symptoms suggesting a potential
vitamin D deficiency, the best way to confirm a diagnosis is through direct measurement of plasma 25(OH)D.2
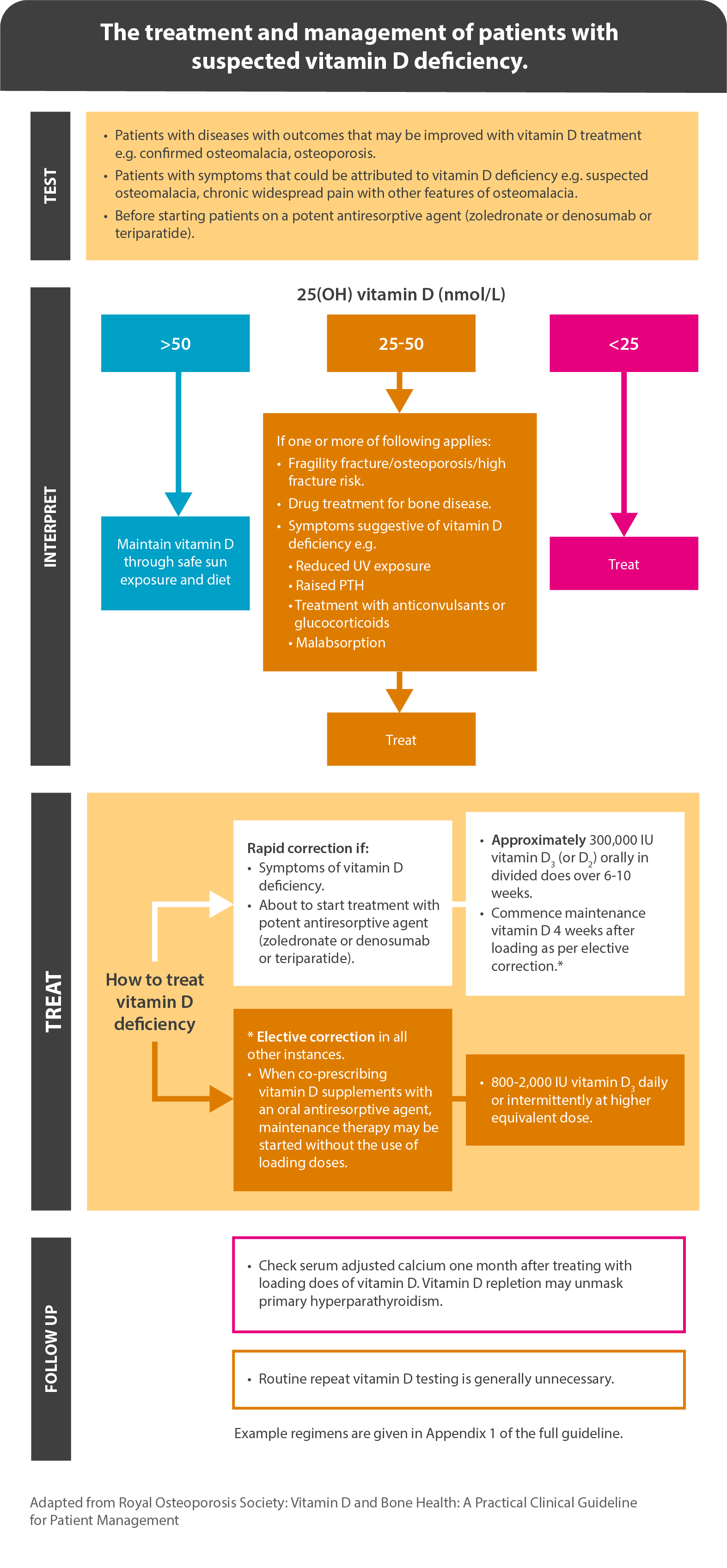
The reference ranges used by the Royal Osteoporosis Society (ROS) are as follows. These ranges also align with thresholds identified by ROS.2
- plasma 25(OH)D < 25 nmol/L is deficient.
- plasma 25(OH)D of 25–50 nmol/L may be inadequate in some people.
- plasma 25(OH)D > 50 nmol/L is sufficient for almost the whole population.
The Royal Osteoporosis Society has developed a quick guide for the treatment and management of patients with suspected vitamin D deficiency.2


 Deutschland
- Deutsch
Deutschland
- Deutsch España
- Español
España
- Español Italia
- Italiano
Italia
- Italiano