Efficacy of MOVENTIG in treating opioid-induced constipation (OIC)2
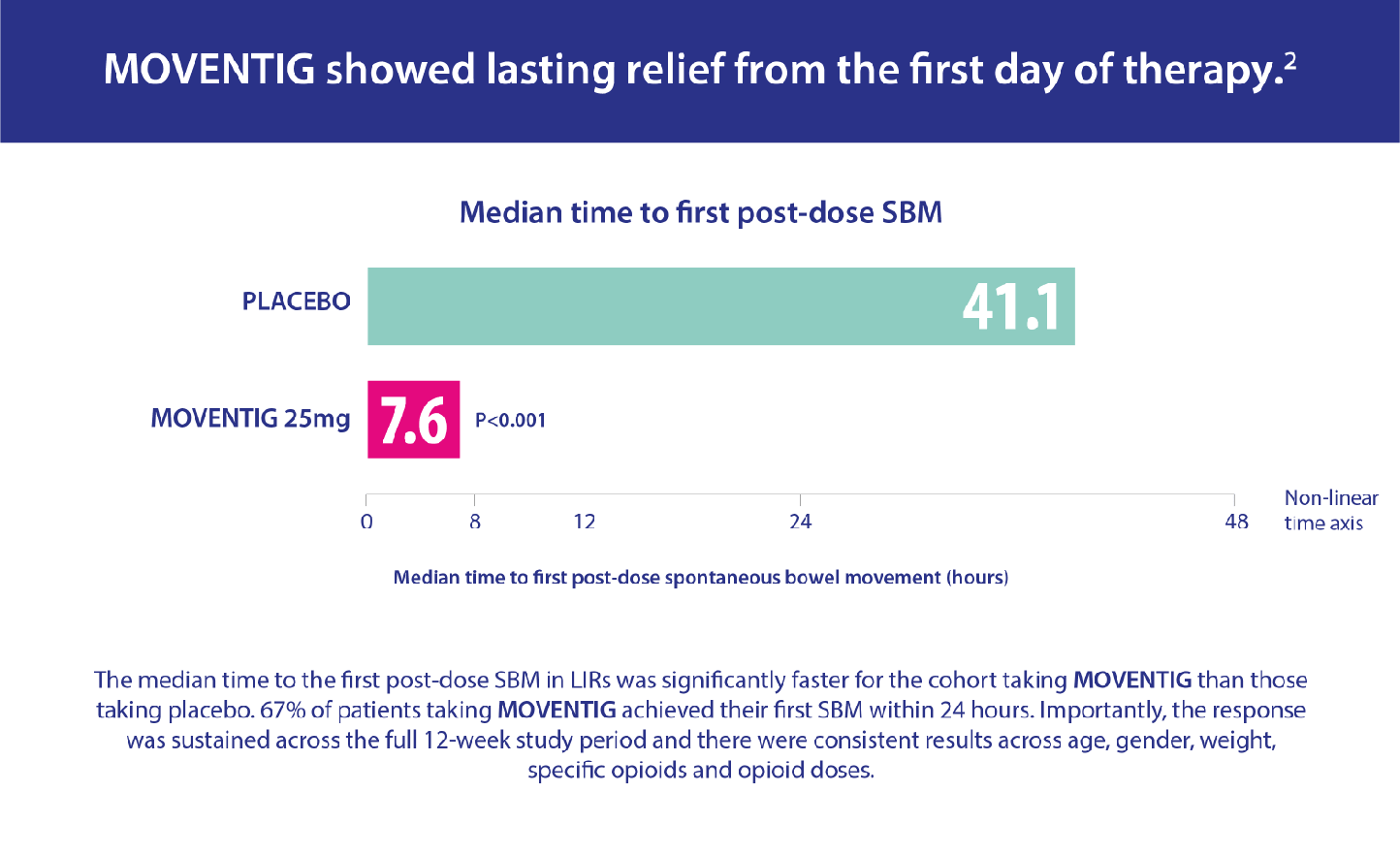
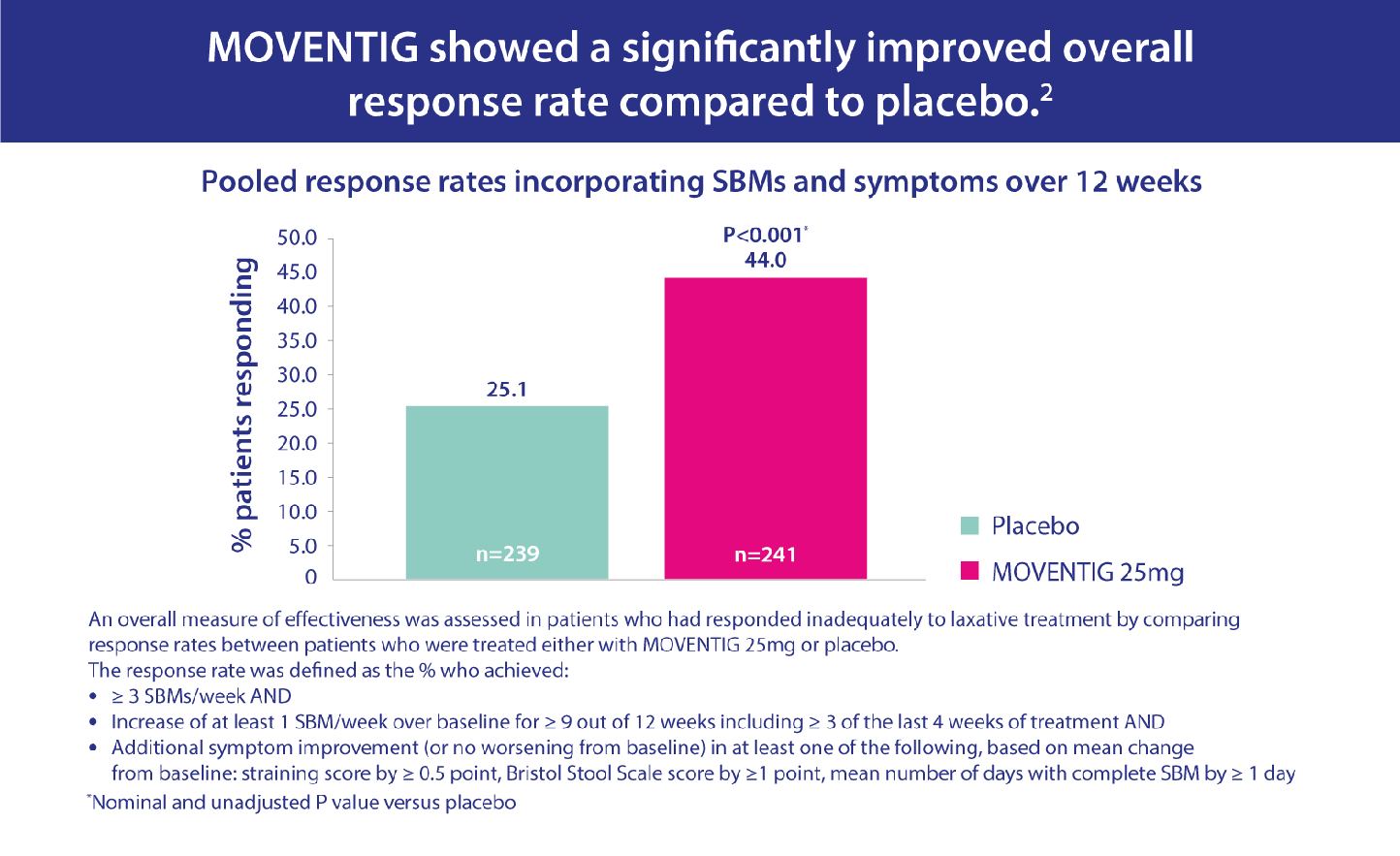
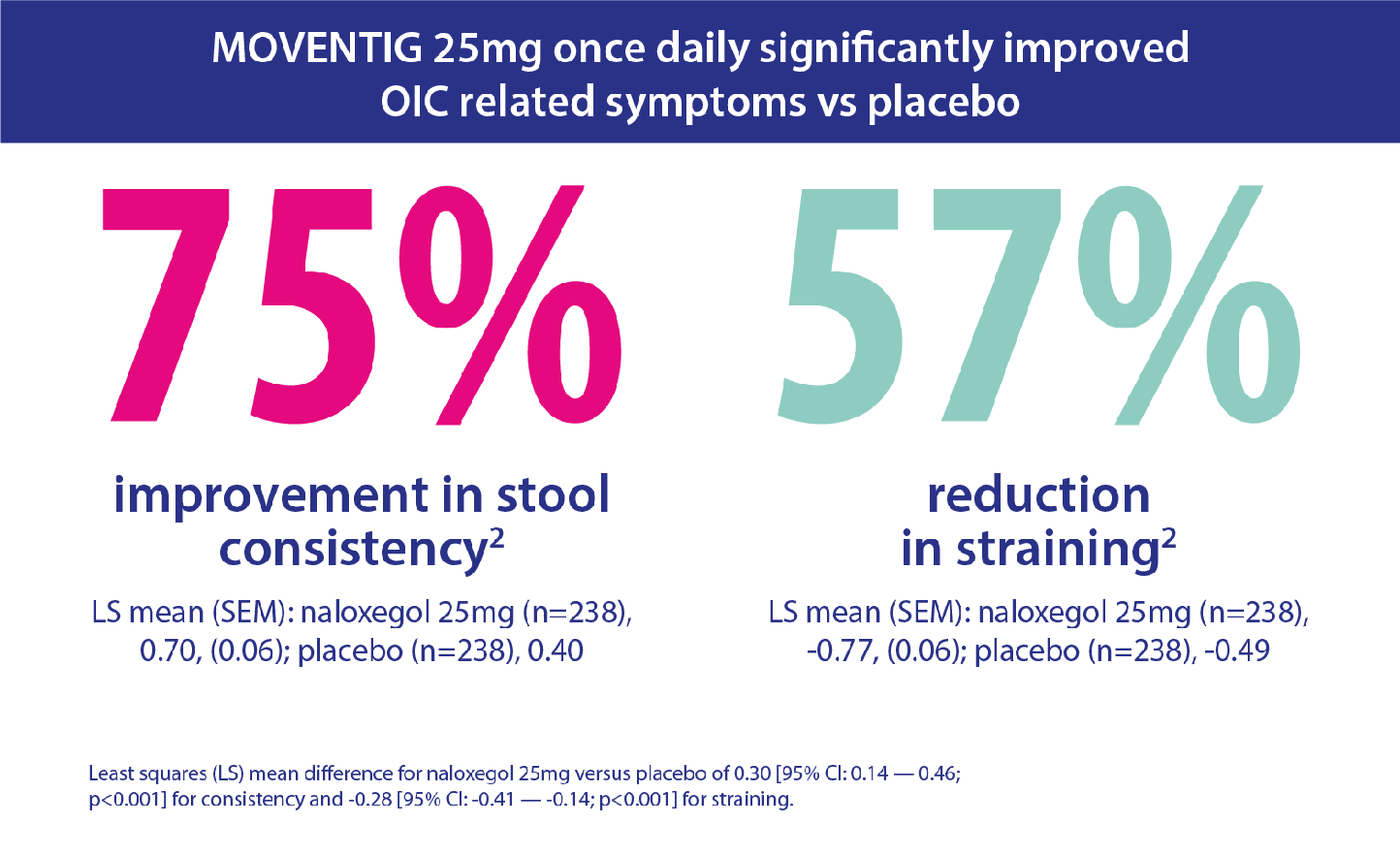
The efficacy of MOVENTIG has been compared in clinical trials to placebo in treating patients suffering from OIC who have had an inadequate response to laxative therapy.
Pooled data from two randomised, double-blind, placebo-controlled trials were analysed. The patients were experiencing non-cancer pain, aged 18–84 with OIC and had inadequate response to laxatives (defined as concurrent OIC symptoms of at least moderate severity while taking at least one laxative class for a minimum of four days during the pre-study period).1 Patients were randomised to receive MOVENTIG 12.5 mg, 25 mg or placebo. Assessments included response rate, time to first post-dose spontaneous bowel movement, number of spontaneous bowel movements (SBMs), OIC symptoms and patient-reported outcomes over 12 weeks.


 Deutschland
- Deutsch
Deutschland
- Deutsch España
- Español
España
- Español Italia
- Italiano
Italia
- Italiano