MOVENTIG in treating opioid-induced constipation (OIC) in patients with cancer2
MOVENTIG in routine clinical practice has been studied in a cohort of patients with cancer. This was a real world study with no comparator group, where patients' mean scores were compared to mean scores at baseline.
- Patients (n=126), mean age 61.5 years, with cancer, on treatment with opioids for pain control and OIC with an inadequate response to laxatives. (LIR, defined as patients with concurrent OIC symptoms of at least moderate severity while taking at least one laxative class for a minimum of four days during the pre-study period.)1
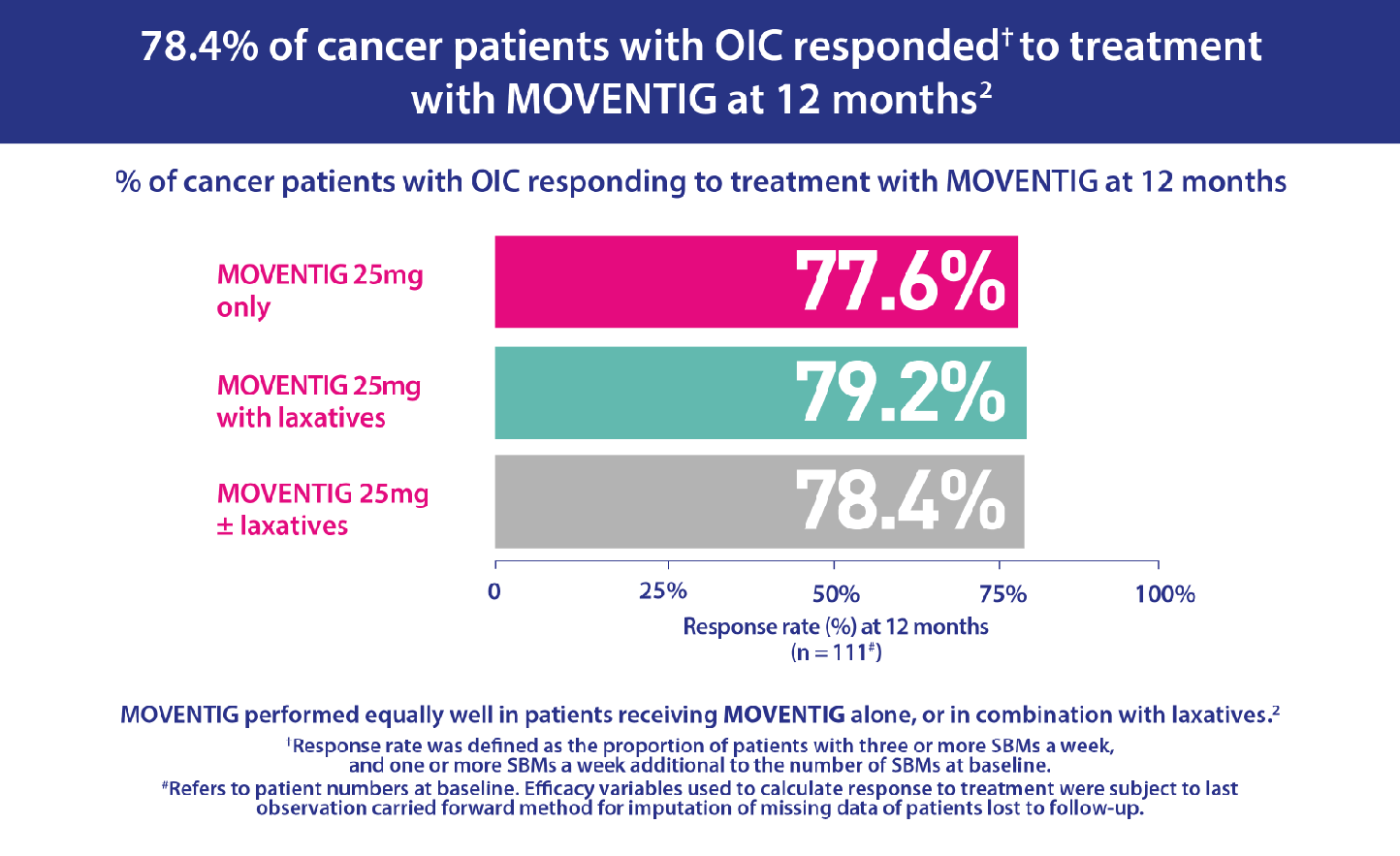
- The starting dose of naloxegol was 25mg/day in 88.1% (n=111) of patients. The naloxegol dose was maintained unchanged during the study in 98.4% (124) of patients.
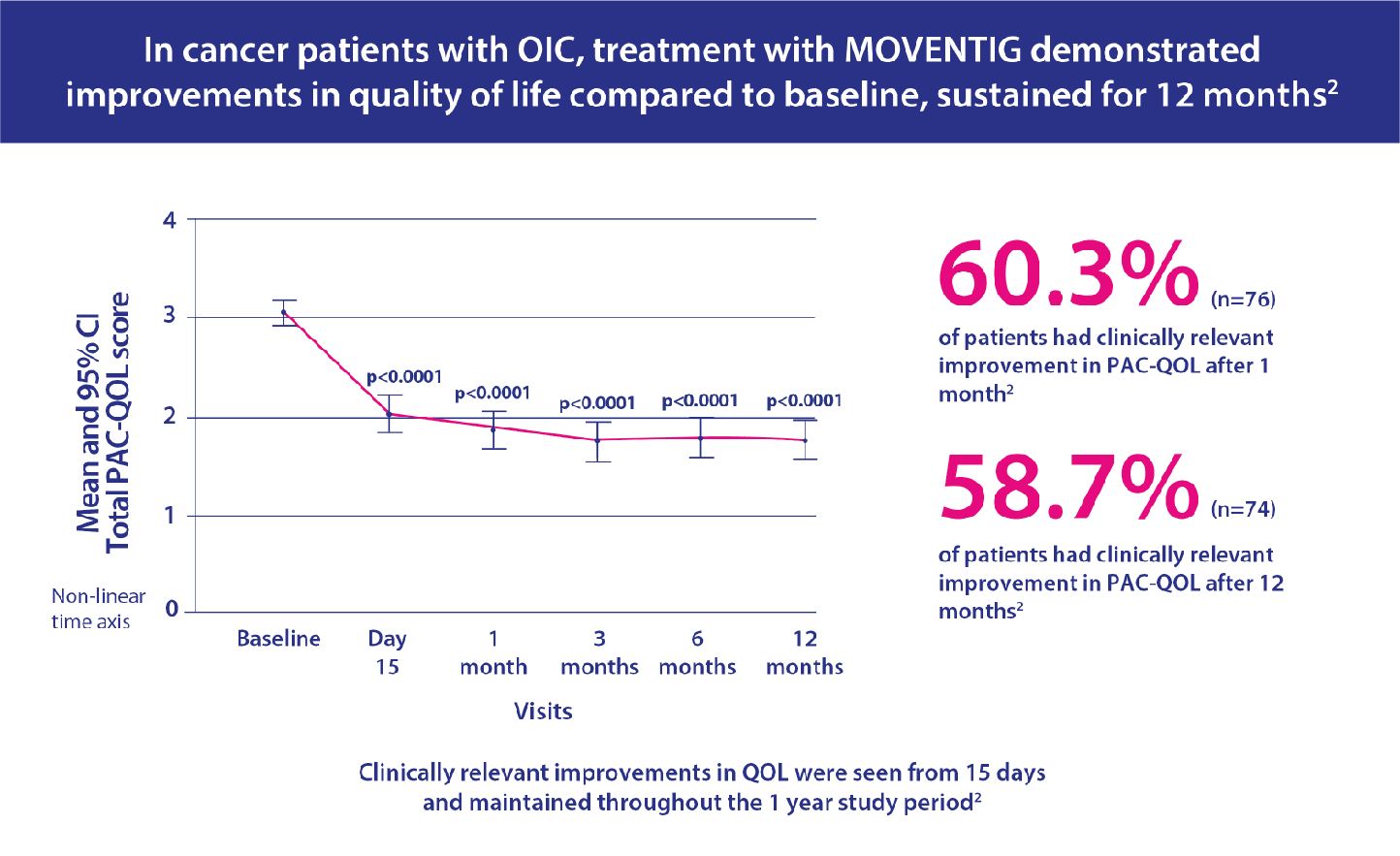
- Primary endpoint was effect on constipation-related quality of life measured by Patient Assessment of Constipation Quality of Life Questionnaire (PAC-QOL).
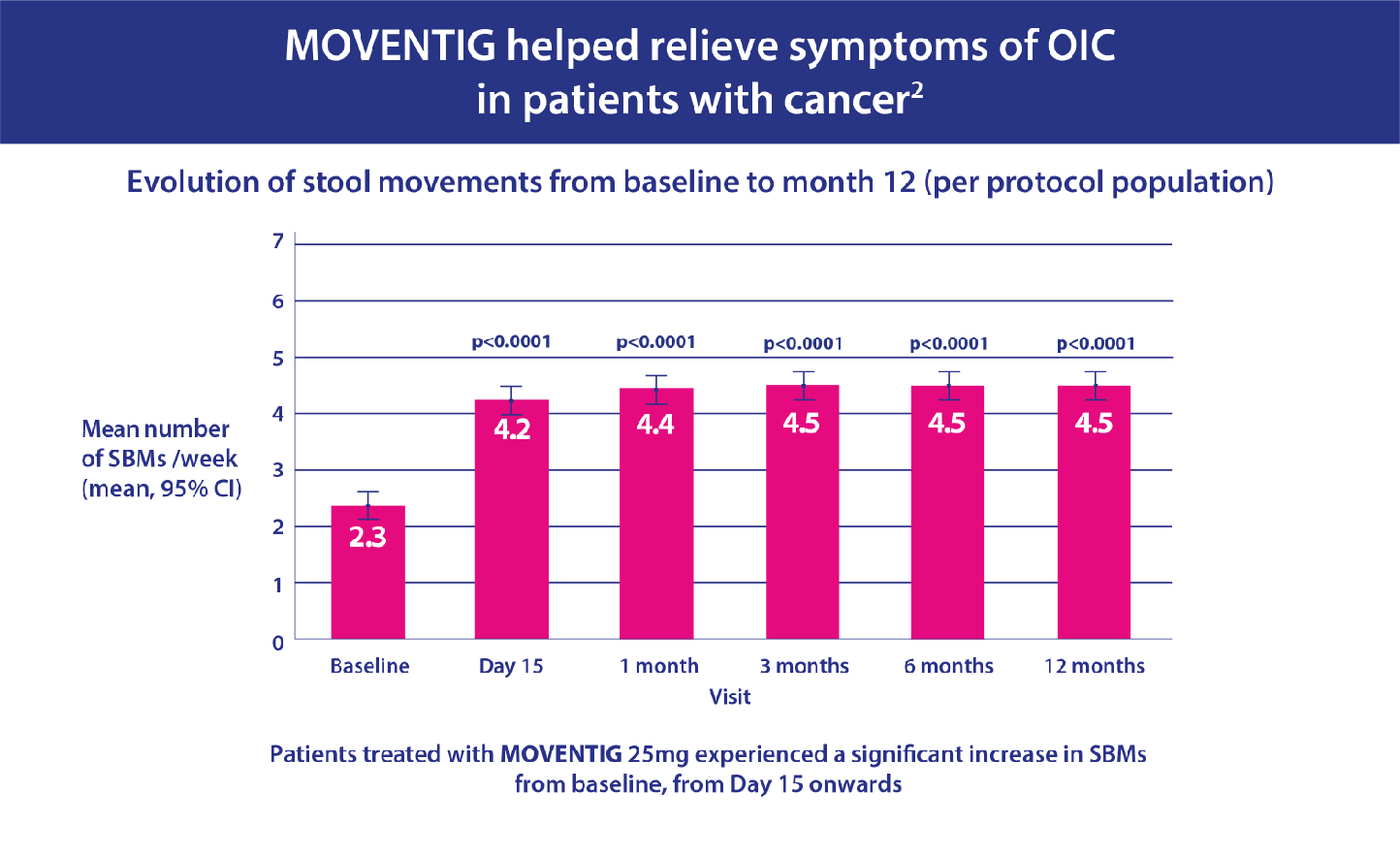
- Secondary endpoints included effect on spontaneous bowel movements (SBMs) during follow-up.
- Adverse reactions were observed in 15.1% of patients (19/126), most appeared in the first 15 days of treatment and resolved with time.2


 Deutschland
- Deutsch
Deutschland
- Deutsch España
- Español
España
- Español Italia
- Italiano
Italia
- Italiano